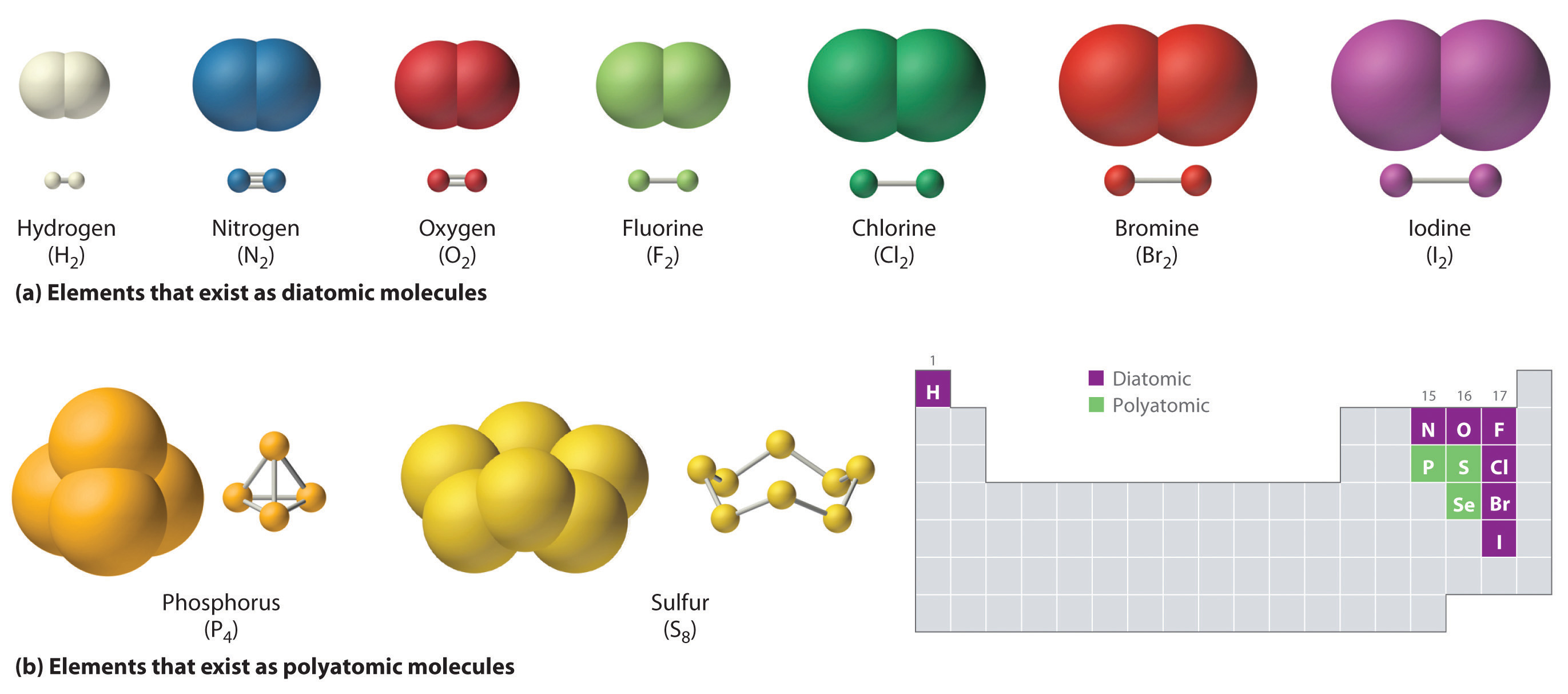
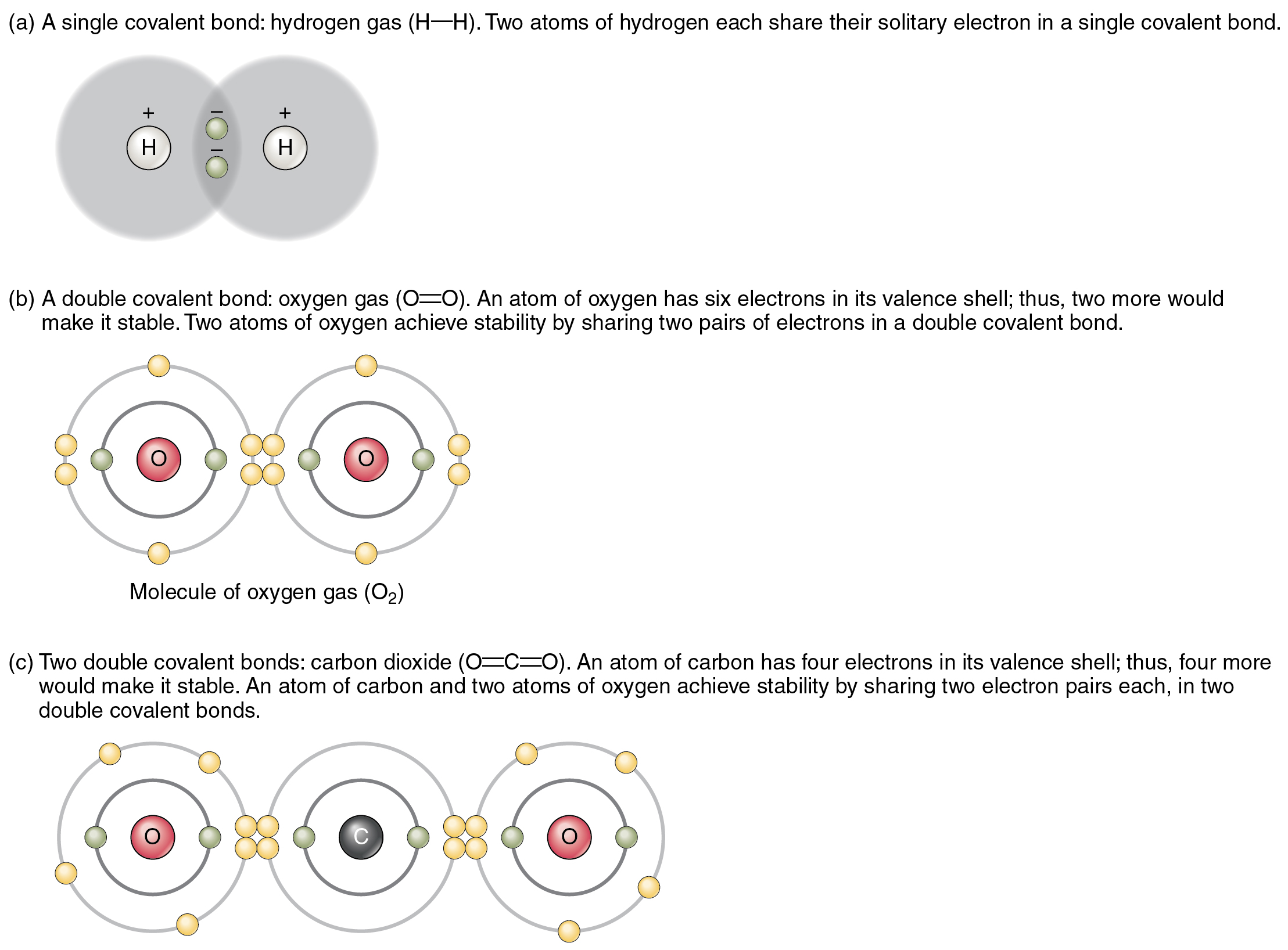
How Many Covalent Bonds Can Phosphorus Form - It has 5 valence electrons, which means that. Therefore phosphorus maximum covalency of 6. Web hydrogen chloride the hydrogen has a helium structure, and the chlorine an argon structure. Web how many covalent bonds is phosphorus? Methane gas (ch 4) has a nonpolar covalent bond because it is a gas. Is phosphorus ionic or convalent. Web there are many different modifications of phosphorus in nature. Group 5a form 3 bonds; With increasing thermodynamic stability they. Web the number refers to the number of bonds each of the element makes:
covalent bond Definition, Properties, Examples, & Facts Britannica
Web phosphorus s2p3 has a 5 valence electrons and needs three more to complete the rule of octet. Web a covalent bond is the same as a ionic bond. In the case of phosphorus, 5 covalent bonds are possible. Web how wants phosphorus form 5 covalent bonds? Web phosphorus is a nonmetallic element having an atomic number of 15.
DNA is made up of phosphate groups, nitrogen bases, and what? Socratic
And that will most likely be the case. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web nitrogen, carbon, and phosphorus can all form triple covalent bonds. Web typically, the atoms of group 4a form 4 covalent bonds; Group 6a form 2 bonds;
Components and Structure OpenStax Biology 2e
With increasing thermodynamic stability they. Group 5a form 3 bonds; Phosphorus only 'needs' three more electrons to get a full valence shell of eight, but you'll notice. Web nitrogen, carbon, and phosphorus can all form triple covalent bonds. Web as such, the \(\ce{s}\) atom must have 12 valence shell electrons to form 6 covalent bonds.
Facts About Phosphorus Live Science
A molecule is nonpolar if the shared electrons are are equally shared. It has 5 valence electrons, which means that. Phosphorus only 'needs' three more electrons to get a full valence shell of eight, but you'll notice. It initiates with a simple picture concerning of single cadmium. Web the number refers to the number of bonds each of the element.
CH103 Chapter 5 Covalent Bonds and Introduction to Organic Molecules
Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Web nitrogen and phosphorus have unpaired electron(s) in their outer shell and, therefore, form covalent bond(s) with other atoms. Web nitrogen, carbon, and phosphorus can all form triple covalent bonds. Group 5a form 3 bonds; Web as such, the \(\ce{s}\).
Covalent Bonds Biology for Majors I
Web on average, phosphorus can form up to three covalent bonds with other atoms (depending on its electron. Web nitrogen and phosphorus have unpaired electron(s) in their outer shell and, therefore, form covalent bond(s) with other atoms. Web consistent with the metastable condition of the white modification, and the crowding of its covalent bonds, this form. Web how many bonds.
Chemical Bonds · Anatomy and Physiology
Web on average, phosphorus can form up to three covalent bonds with other atoms (depending on its electron. Web in general your assumption is correct, that it is possible to form only three covalent bonds to reach a stable configuration. And that will most likely be the case. Web there are many different modifications of phosphorus in nature. Web typically,.
Chemical Bonding Types of Chemical Bonds, Bond Characteristics, Enthalpy
Web how many bonds does phosphorus typically make? Chlorine s2p5 has 7 valence. Similarly, the phosphorus atom in. The octet rule only applys to molecules with covalent bonds. Web the number refers to the number of bonds each of the element makes:
Chemical Bonds Anatomy and Physiology I
Methane gas (ch 4) has a nonpolar covalent bond because it is a gas. Web as such, the \(\ce{s}\) atom must have 12 valence shell electrons to form 6 covalent bonds. Web phosphorus s2p3 has a 5 valence electrons and needs three more to complete the rule of octet. Web phosphorus is a nonmetallic element having an atomic number of.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
And that will most likely be the case. Therefore phosphorus maximum covalency of 6. Web hydrogen chloride the hydrogen has a helium structure, and the chlorine an argon structure. Group 6a form 2 bonds; Web consistent with the metastable condition of the white modification, and the crowding of its covalent bonds, this form.
It initiates with a simple picture concerning of single cadmium. Web how many covalent bonds is phosphorus? In the case of phosphorus, 5 covalent bonds are possible. Methane gas (ch 4) has a nonpolar covalent bond because it is a gas. Web consistent with the metastable condition of the white modification, and the crowding of its covalent bonds, this form. Web nitrogen, carbon, and phosphorus can all form triple covalent bonds. Therefore phosphorus maximum covalency of 6. Web the number of electrons required to obtain an octet determines the number of covalent bonds an atom can form. Chlorine s2p5 has 7 valence. Web in general your assumption is correct, that it is possible to form only three covalent bonds to reach a stable configuration. Web how wants phosphorus form 5 covalent bonds? Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and. Web phosphorus s2p3 has a 5 valence electrons and needs three more to complete the rule of octet. Is phosphorus ionic or convalent. Web nitrogen and phosphorus have unpaired electron(s) in their outer shell and, therefore, form covalent bond(s) with other atoms. Posted on ankush sharma 17 may 2020 11 mar 2021 posted inside. It has 5 valence electrons, which means that. A molecule is polar if the shared electrons are equally shared. Similarly, the phosphorus atom in. Web typically, the atoms of group 4a form 4 covalent bonds;
Web A Covalent Bond Is The Same As A Ionic Bond.
Methane gas (ch 4) has a nonpolar covalent bond because it is a gas. Web hydrogen chloride the hydrogen has a helium structure, and the chlorine an argon structure. And that will most likely be the case. The octet rule only applys to molecules with covalent bonds.
Web The Number Of Valence Electrons In Phosphorus Is Generally 5 And It Requires 3 More Electrons To Complete Its.
Group 5a form 3 bonds; Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and. Web on average, phosphorus can form up to three covalent bonds with other atoms (depending on its electron. Web how many bonds does phosphorus typically make?
Web Phosphorus Is A Nonmetallic Element Having An Atomic Number Of 15.
Similarly, the phosphorus atom in. Web consistent with the metastable condition of the white modification, and the crowding of its covalent bonds, this form. A molecule is polar if the shared electrons are equally shared. Therefore phosphorus maximum covalency of 6.
Posted On Ankush Sharma 17 May 2020 11 Mar 2021 Posted Inside.
Web as such, the \(\ce{s}\) atom must have 12 valence shell electrons to form 6 covalent bonds. In the case of phosphorus, 5 covalent bonds are possible. Web typically, the atoms of group 4a form 4 covalent bonds; Web in both the red and the black forms, each phosphorus atom forms three single bonds, which are spread apart sufficiently to be.